Abstract
Background: Advances in the treatment of aplastic anemia (AA), such as the addition of eltrombopag (EPaG) to immunosuppressive therapy (IST) and the expanded use of unrelated and haploidentical stem cell transplant (SCT) have steadily improved outcomes. However, little is known about the characteristics and outcomes of patients (pts) ≥ 60 years (yrs) of age with newly diagnosed AA who have been treated with an ATG-based regimen. Presumed low bone marrow (BM) reserves, higher frequency of underlying clonal hematopoiesis, intolerance to therapy, comorbidities, and challenges in proceeding to salvage SCT have led to the speculation that response and survival of these pts to IST may be inferior than their younger counterparts.
Methods: We retrospectively analyzed the characteristics and outcomes of pts ≥ 60 yrs of age (OLDER) at the time of diagnosis of AA treated with frontline (F/L) ATG based therapy at our center and compared them to pts < 60 yrs of age (YOUNG) treated with F/L ATG over the same time period. We captured their clinical characteristics, cytogenetic abnormalities, mutations in DNMT3A (D), ASXL1 (A) and TET2 (T), exposure to EPaG, response, time to response (TTR) and survival. Overall response included complete and partial response per the standard definitions. Relapse free survival (RFS) was tabulated from the time of best response to loss of response, 2nd ATG infusion, salvage SCT or death (whichever was earlier) and overall survival was tabulated from the time of response to death from any cause.
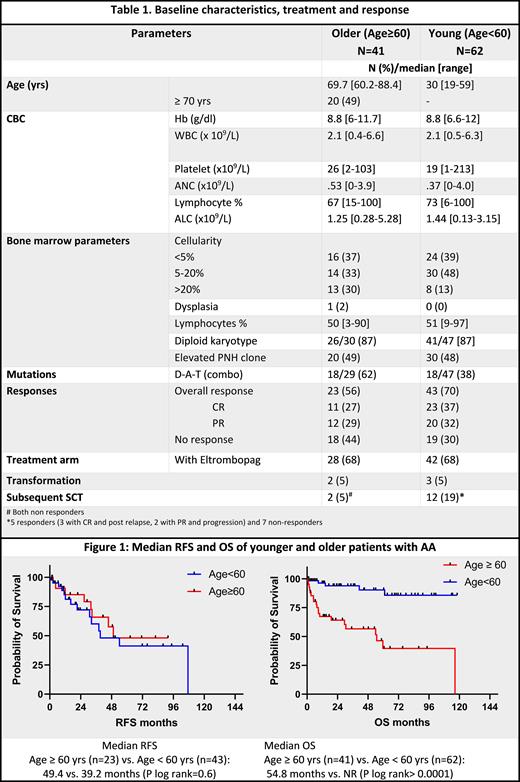
Results: From Jan 2011-May 2012, 43 OLDER pts were treated as F/L for AA of whom 41 (95%) received ATG based therapy; 68 YOUNG pts were treated as F/L for AA over the same time period of whom 62 (91%) received ATG based regimen. Four YOUNG pts vs. no pts in the OLDER group received upfront SCT, and 2 pts in each group were treated with non ATG based therapy: single agent CSA, EPaG, or alemtuzumab. Comparative baseline characteristics are depicted in Table 1. There were no differences in the baseline WBC, peripheral blood lymphocyte percentage and platelet counts between the ATG treated OLDER and YOUNG groups. Both groups had similar frequencies of cytogenetic abnormalities and PNH clones, though OLDER pts had mutations in D-A-T genes compared to the YOUNG group (62% vs. 38%, p=0.05). Both groups had equal concurrent exposure to EPaG with ATG. Cumulatively 23/41 (56%) pts in the OLDER group had an overall response (OR) compared to 43/62 (70%) pts in the YOUNG group (p=0.1); 11/23 (48%) and 23/43 (53%) responders in each group attained CR respectively. There were no differences in OR rates in both groups based on concurrent EPaG exposure: 61% vs. 46% with and without EPaG, respectively, in the OLDER group (p=0.5) and 74% vs 60%, respectively, (p=0.38) in the YOUNG group. On logistic regression analysis using baseline platelet counts, PB lymphocyte (%), D-A-T mutations, karyotype, and EPaG use as covariates, none affected the odds of OR in the 2 groups. The median TTR was 111 days (range, 10-323 days) and 177 days (51-410) in YOUNG AND OLDER groups, respectively; and this was not affected by EPaG use in either group.
At a median follow of 38 months (mos) from ATG initiation (53 mos for the OLDER group and 32 mos for the YOUNG group), the median RFS was 49 mos and 39 mos (p=0.6) and median OS 55 vs. not reached (NR) (p <0.0001) in OLDER and YOUNG groups respectively (Fig.1). Significantly more pts in the YOUNG group underwent a subsequent SCT (19% vs. 5%) due to loss of response/ no response, possibly accounting for the higher OS rates. Rates of transformation to MDS or AML was 5% in both groups.
Conclusion: Frontline ATG based IST in older pts with AA is a feasible and safe treatment option with promising response rates of 56% and median RFS more than 4 years; figures which are comparable to younger pts with AA treated with IST. The overall risks of transformation to MDS/AML on long term follow-up remain low (≈5%). Further studies assessing the response of the ATG+EPaG combination in these pts and the possibilities of clonal evolution with time needs to be conducted.
Disclosures
Kantarjian:Pfizer: Honoraria, Research Funding; Jazz Pharmaceuticals: Research Funding; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Research Funding; ImmunoGen: Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Research Funding; NOVA Research: Honoraria; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Takeda: Honoraria. Ravandi:Amgen: Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Biomea Fusion, Inc.: Research Funding; Prelude: Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Xencor: Research Funding; Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Syos: Consultancy, Honoraria, Research Funding; Novartis: Consultancy; AstraZeneca: Consultancy. DiNardo:GenMab: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Kura: Honoraria, Membership on an entity's Board of Directors or advisory committees; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding; Servier: Consultancy, Honoraria, Research Funding; Astellas: Honoraria; Bluebird Bio: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Foghorn: Honoraria, Research Funding; ImmuneOnc: Honoraria, Research Funding; LOXO: Research Funding; Novartis: Honoraria; Takeda: Honoraria; Astex: Research Funding; Cleave: Research Funding; Forma: Research Funding; Gilead: Honoraria; Jazz: Honoraria. Takahashi:Illumina: Honoraria; Symbio Pharmaceuticals: Consultancy; Agios: Consultancy; Celgene/BMS: Consultancy; GSK: Consultancy; Mission Bio: Honoraria; Ostuka Pharmaceuticals: Honoraria; Novartis: Consultancy. Sasaki:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Otsuka Pharmaceuticals: Honoraria. Kanagal-Shamanna:Amgen: Consultancy; Novartis: Consultancy; Aptitude Health: Speakers Bureau; Physicians Education Resource: Speakers Bureau. Popat:Iovance: Consultancy; Bayer: Research Funding; Incyte: Research Funding; Abbvie: Research Funding; Novartis: Research Funding. Jabbour:Adaptive Biotechnologies: Other: Advisory Role, Research Funding; Amgen: Other: Advisory Role, Research Funding; Pfizer: Other: Advisory Role, Research Funding; Bristol Myers Squibb: Other: Advisory Role, Research Funding; Genentech: Other: Advisory Role, Research Funding; Spectrum: Research Funding; AbbVie: Other: Advisory Role, Research Funding; Takeda: Other: Advisory Role, Research Funding. Champlin:Johnson &Johnson: Consultancy; Actinium: Consultancy; Kadmon: Consultancy; Omeros: Consultancy; Bluebird: Other: Data Safety Monitoring Board; General Oncology: Other: Data Safety Monitoring Board; Cell Source Inc.: Research Funding. Garcia-Manero:Genentech: Honoraria, Research Funding; Astex: Consultancy, Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Curis: Honoraria, Research Funding; Aprea: Honoraria; BMS: Consultancy, Honoraria, Research Funding; Gilead Sciences: Research Funding; Acceleron Pharma: Consultancy. Kadia:Astex: Honoraria; Servier: Consultancy; Regeneron: Research Funding; Glycomimetics: Research Funding; Astellas: Research Funding; JAZZ: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; cyclacel: Research Funding; Delta-Fly: Research Funding; PinotBio: Consultancy; cellenkos: Research Funding; Ascentage: Research Funding; Genfleet: Research Funding; Pfizer: Research Funding; Novartis: Consultancy; Amgen: Research Funding; AstraZeneca: Research Funding; BMS: Consultancy, Research Funding; Agios: Consultancy; Iterion: Research Funding; Abbvie: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal